Abstract
Background: Engineered toxin bodies (ETBs) are comprised of a proprietarily engineered form of Shiga-like Toxin A subunit (SLT-A) genetically fused to antibody-like binding domains. ETBs are capable of forcing internalization, self-routing through intracellular compartments to the cytosol, and inducing potent cell-kill via the enzymatic and permanent inactivation of ribosomes. MT-0169 is a first in class de-immunized ETB targeting CD38 for hematological tumors, including multiple myeloma (MM) and non-Hodgkin lymphoma. MT-0169 may not be subject to resistance mechanisms that exist for other CD38-targeted therapies.
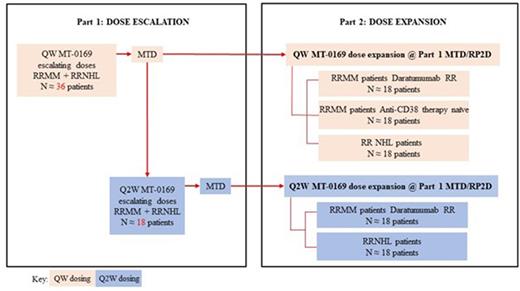
Trial Design: This is a multicenter, open-label, phase 1 study designed to evaluate the safety, tolerability, preliminary efficacy, pharmacokinetics (PK), pharmacodynamics, and immunogenicity of MT-0169 monotherapy in patients with relapsed or refractory multiple myeloma (RRMM) or relapsed or refractory Non-Hodgkin lymphoma (RRNHL). The primary endpoint of the dose escalation phase (Part 1) is to evaluate the safety and tolerability of MT-0169 monotherapy in patients with RRMM and NHL and establish the maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D); the primary endpoint of the dose expansion phase (Part 2) is to provide a preliminary evaluation of the clinical activity of MT-0169 monotherapy in patients with RRMM and RRNHL.
Both parts of the study are enrolling RRMM and RRNHL patients who are ≥18 years old and who meet the disease specific standards for refractory/relapsed disease. RRMM patients should have received ≥3 prior lines of therapy including at least 1 proteasome inhibitor (PI), 1 immunomodulatory drug (IMiD), and 1 steroid (or ≥2 prior lines if 1 of those lines included a combination of PI and IMiD). Prior treatment with an anti-CD38 therapy (including daratumumab) is permitted. RRNHL patients are eligible if they have failed treatment with, are intolerant to, or are determined by the investigator not to be a candidate for available therapies that are considered standard of care or are known to confer clinical benefit. In Part 1, MT-0169 will be administered by IV infusion once weekly (QW) on Days 1, 8, 15, and 22 of each 28-day cycle. Following the determination of the QW MTD, a second dose escalation cohort evaluating once every 2 weeks (Q2W) dosing (Days 1 and 15 of each 28-day cycle) may be initiated (Figure 1). Dosing proceeds with 5 μg/kg, 10% of the starting dose, which was 50 μg/kg, and 1/150th of the highest non-severely toxic dose. Currently, patients are being enrolled at dose level (-1) to explore safety. Based on in vitro natural killer cell depletion data and cynomolgus monkey PK/pharmacodynamic study data, this dose is expected to result in pharmacologic activity.
In Part 2, patients with RRMM or RRNHL will receive MT-0169 at the QW or Q2W MTD/RP2D that is available from Part 1. The RRMM expansion cohorts will evaluate 2 types of RRMM patients in 3 cohorts: Daratumumab-relapsed or refractory (RR) cohorts (QW and Q2W MT-0169 administration) and an anti-CD38 Therapy Naïve cohort (QW MT-0169 administration). Patients will continue treatment with MT-0169 until they experience progressive disease, unacceptable toxicity, or withdraw for other reasons. The trial is currently recruiting at multiple sites in the United States. (NCT04017130).
Disclosures
Dholaria:Jazz Pharmaceuticals: Consultancy; Pfizer: Research Funding; Poseida: Research Funding; Angiocrine: Research Funding; Arivan: Consultancy; Molecular Templates: Research Funding; MEI Pharma: Research Funding; BMS: Research Funding; Takeda: Research Funding; Orca Bio: Research Funding; Janssen: Research Funding; BEAM Therapeutics: Consultancy; Gamida Cell: Consultancy; MJH Biosciences: Honoraria; Vanderbilt University Medical Center: Current Employment; Wugen: Research Funding. Mamuye:Molecular Templates, Inc.: Current Employment. Yurewicz:Molecular Templates, Inc.: Current Employment. Dabovic:Molecular Templates, Inc.: Current Employment. Yuet:Molecular Templates, Inc.: Current Employment. Abonour:Amgen: Honoraria; Takeda: Honoraria, Research Funding; Janssen: Honoraria, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Prothena: Honoraria. Kelly:Novartis: Honoraria, Speakers Bureau; Pharmacyclics: Honoraria, Speakers Bureau; Spectrum: Consultancy; Genoptix: Consultancy; Celgene: Honoraria; Epizyme: Honoraria; Karyopharm: Honoraria; GSK: Honoraria; BMS: Honoraria; Seattle Genetics: Honoraria; Gilead: Honoraria; Verastem: Consultancy; AstraZeneca: Consultancy; Sanofi-Aventis: Consultancy; Denovo Biopharm: Consultancy; Amgen: Consultancy; Takeda: Research Funding; Oncolytics Biotech Inc: Research Funding; Berkley Lights: Current equity holder in private company; Agios: Current equity holder in private company. Voorhees:AstraZeneca: Research Funding; Incyte: Research Funding. Kazandjian:Plexus Communications: Honoraria; Aptitude Health: Honoraria; Curio Science: Honoraria; MMRF: Honoraria; Arcellx: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; SINTOMA: Honoraria; CURE: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal